Additional technologies you may want to consider
Fertility technology you may to consider with IVF
Fertility technology you may to consider want with IVF:
Embryo Freezing
Often more than two or three embryos are produced in an IVF cycle. Good quality 'spare' embryos can be frozen, and later thawed to give another chance of pregnancy. Freezing and thawing have to be done under special conditions: >90% of embryos can survive the procedure. When embryos are to be thawed the woman's menstrual cycle is monitored with blood tests to make sure the embryos are replaced at the right time of the menstrual cycle.
Thaw cycle of IVF
When there have been embryos frozen following in vitro fertilisation, they can be thawed and transferred into the uterus at a later stage. This usually entails several bloods tests to time ovulation during a natural cycle. In some cases it is necessary to control a cycle using drugs to prepare the uterus for implantation. Embryos are transferred into the uterus at the appropriate time, as they are in an IVF cycle.
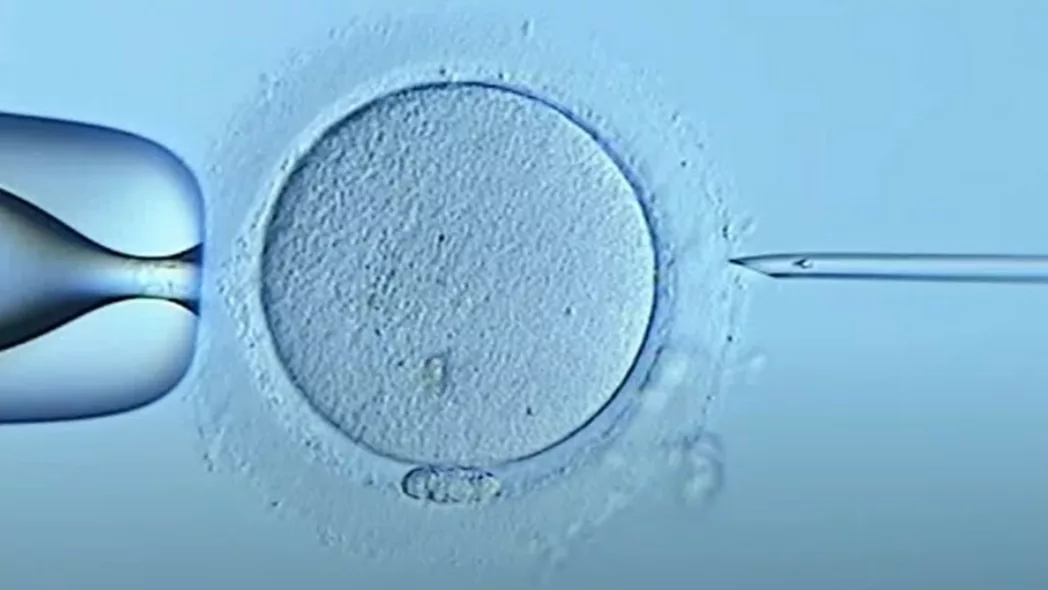
ICSI
Intra-cytoplasmic sperm injection, or ICSI, is a procedure in which a single sperm is manually injected directly into an egg. Once fertilised successfully, the eggs will grow and divide for three to five days to create embryos, exactly like conventional IVF. An embryo is then transferred into the woman’s uterus, with the aim of resulting in a pregnancy.
ICSI procedure
This treatment is very similar to IVF. The main difference is that instead of mixing the sperm with the eggs and leaving them to fertilize, a skilled embryologist will inject a single sperm into the egg. This maximizes the chance of fertilization taking place as it bypasses any potential problems the sperm may have in getting inside the egg.
Procedure in steps:
Step 1: Sperm collection
Step 2: Egg collection
Step 3: Sperm injection
Step 4: Observation
Step 5: Implantation
- Before ICSI can be carried out, mature eggs must be collected during a standard stimulated IVF cycle.
- After the eggs are collected, the outer coating of cells (cumulus cells) is removed from each egg. Only then can we be sure the egg is mature enough to undergo the ICSI procedure.
- Immature eggs cannot be injected. However, they can be incubated for a further two to six hours and reassessed. If they mature in that time, they can still be injected along with the other mature eggs. Patients are then notified on the following day as to how many eggs were mature and how many were injected.
- The semen sample is prepared in the lab to isolate healthy, moving sperm for the ICSI procedure. During this process we remove any debris, dead cells and seminal plasma.
- Moving sperm are selected for the ICSI procedure based on their morphology (shape), however, this visual approach may not necessarily reflect their functionality or ability to fertilise an egg.
- A very fine special glass instrument, (called a holding pipette), is used to hold the egg still. It is so small you can barely see the tip with the naked eye. A thinner, sharp, glass needle-like instrument is then used to immobilise and pick up a single sperm.
- With great care and precision, the needle is gently inserted through the egg’s outer shell, (the zona pellucida), and into the egg itself. The immobilised sperm is then slowly injected into the egg and the needle is carefully removed, leaving the sperm inside the egg.
- The injected eggs are placed in an incubator overnight and checked the next morning for signs of fertilisation. After an additional 24 hours, we can start to determine how many have divided and gone on to form embryos.
It is important to note that not all eggs will fertilize, and not all fertilized eggs become embryos. As with standard IVF, the number of embryos transferred into the uterus depends on the woman’s age and medical history. Provided the additional embryos appear healthy, and are progressing at the expected rate of growth, they can also be frozen and stored for future use.
Who is ICSI recommended for?
ICSI is recommended for couples who have had poor or no fertilization during standard IVF, as well as men who have:
- Poor sperm morphology (abnormally shaped sperm).
- Poor sperm motility (slow moving).
- A low sperm count.
- An obstruction such as a vasectomy, which prevents sperm release.
- Antisperm antibodies (antibodies that are produced by a man’s body and may inhibit sperm function).
- A vasectomy reversal that was unsuccessful or resulted in a low sperm count or poor-quality sperm.
What are potential risks associated with ICSI?
For the egg:
As ICSI is more invasive and requires more handling time than standard IVF insemination techniques, there is a small chance (less than 2%) that the egg may be damaged during the procedure – resulting in a non-viable egg.
For the resulting child:
Thousands of children around the world have been born as a result of ICSI. So far, there is no convincing evidence that the incidence of birth defects is any different with ICSI or IVF compared with children born to parents of similar age and health
Genetic Carrier Screening
Genetic carrier screening gives individuals and couple’s information about their risk of having a child with a genetic condition.
We now offer the option to do genetic testing, either for your own information or to check compatibility with your partner or potential donor.
Genetic screening identifies whether you are a carrier of a range of inherited conditions. Most people are carriers of one or more genetic conditions, even though no one in their family has the condition. Being a carrier is important if the person using the sperm is also a carrier of the same condition.
Carrier screening includes:
- Severe and prevalent disorders seen across all ethnicities
- Enhanced SMA testing to help identify silent carriers
- Comprehensive Fragile X analysis, including AGG interruptions
- Full gene sequencing with deletion and duplication analysis leading to a 99% detection rate for most genes
- Actionable results; no reporting of variants of unknown significance
Pre-implantation Genetic Testing (PGT)
Pre-implantation genetic testing can identify genetic abnormalities in embryos at the blastocyst stage, (a blastocyst is an embryo that has been developed in the laboratory for five or six days after fertilisation). This allows us to select the 'best' embryos which are most likely to be free of genetic conditions and chromosomal abnormalities for transfer to the uterus.
Types of PGT:
PGT-M: formerly called PGD, pre-implantation genetic testing for monogenic/single gene disorders. When used by people who have a chance of passing serious genetic disorders on to their children, PGT means that couples can have a child that is fully theirs genetically without the child inheriting the particular genetic disorder. Examples of conditions that may be are caused by a change in single gene may be excluded by this test: neurofibromatosis type 1; Marfan syndrome; cystic fibrosis; Tay Sachs; Huntington's; muscular dystrophy; Beta-thalessemia, Fragile X and Spinal Muscular Atrophy.
PGT-SR: chromosome structural rearrangement (eg translocation carrier). This can assist people who have a high chance of pregnancy loss or a lower chance of pregnancy because of translocations between chromosomes.
PGT-A: aneuploidy testing (formerly called PGS). Because many blastocysts have the wrong number of chromosomes, (a condition known as aneuploidy), PGT is a powerful tool for selecting a good embryo for transfer. Aneuploidy can lead to miscarriages, birth defects and other complications.
Useful to know:
Ethics Committee approval is required to carry out PGD for any other reason, (such as ‘saviour siblings’). PGD cannot be used for gender selection in New Zealand.
TiMI
Photographs embryos every 10 minutes to capture developmental milestones in the embryo’s life that are missed when embryos are only inspected once a day.
Benefits of TiMI:
- Picks up unusual and detrimental events that escape a daily snap-shot inspection.
- Identifies 10-15% of embryos with very low potential.
- Several studies show that TiMI increases the chance of pregnancy using the first embryo transferred by up to 10%.
- Embryos are not removed from their incubator, therefore they develop in an uninterrupted environment.
Who can benefit from TiMI?
- People who expect to have several good quality embryos to choose from.
- People who have had low quality embryos previously and want more information about their embryo development.
Embryo selection with TiMI does not increase the overall chance of a baby from all the embryos available, but it can reduce the time to pregnancy by giving a better choice of which embryo to use first.
How can TiMI & PGT work together?
Preimplantation genetic testing (PGT) is the strongest embryo selection tool we have since we know that many normal appearing embryos have the wrong number of chromosomes (aneuploidy).
The challenge with PGT is that it requires blastocysts on day 5 or 6 for testing – probably 3 or more. TiMI complements PGT because it provides the best undisturbed environment we have available in order to get as many blastocysts as possible. While the extra information we gain from watching the embryos with TiMI is helpful when not using PGS, it does not add to the selection process when PGS is used.
The challenge most patients face is knowing how many embryos they’ll have to work with. If the number is few, then TiMI is a safe way to increase your chances. If there are 3 or more blastocysts, then PGS alone or in combination with TiMI may be the better option.